A brief cover of the paleomicrobiology of Mycobacterium tuberculosis.
Tuberculosis is caused a by a group of
bacteria that are closely phylogenetically related called the
Mycobacterium tuberculosis complex. They are slow growing aerobic
gram positive bacilli that due to its high lipid content in its one
phospholipid bilayer, requires stain methods that facilitate the
dye into their cell wall, such as the heat method of Ziehl Neelsen
staining. It is estimated by the WHO that approximately one third of
the world population have latent TB infections but only 5 to 10
percent of these individuals will develop the disease in their
lifetime.
Skeletal Material
It was recently thought that TB was
likely transferred to humans during the Neolithic period associated
with increasing sedentary lifestyles and domestication of cattle,
perhaps transferred from Mycobacterium bovis (Iseman 2478 : 1994),
but with the exception of Mycobacterium canetti, members of the MTBC
group do not show horizontal gene transfer (Gagneux 852 : 2012) most
of the genetic differences in the MTBC are due to deletions. Thus
comparison of human M tuberculosis and M bovis indicate that the M
bovis genome is around 60 000 base pairs smaller than the human M
tuberculosis genome, thus the human M tb lineage is older than M
bovis and this also suggest TB in humans predates the Neolithic
period (Gagneux 852 : 2012).
TB in the bone is the result of a post
primary tuberculosis spread and is a chronic process, thus features
remodelled marginal erosive lesions. In cases of active TB, 3 to 5 %
of individuals develop bone lesions (Holloway et al 2011). Newly
formed woven bone can be distinguished from mature lamellar sclerotic
changes, that can suggest healing. The presence of woven bone
indicates lesions active at time of death while sclerotic changes
changes signal an inactive disease process (Roberts & Buikstra 88
: 2003). Mycobacterium bovis, is more likely to cause skeletal damage
and is potentially up to 20 times more likely to lead to bone and
joint tuberculosis in children.
Other diseases that show up in skeletal
material include osteomyelitis, syphilis and leprosy. Indicated in Figure A.
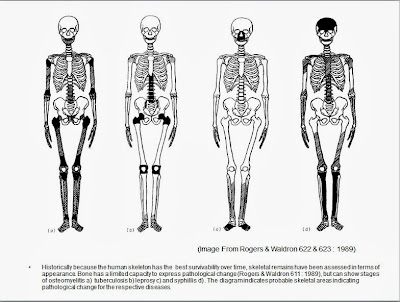 |
| Figure A |
Because these diseases leave evidence
in skeletal material much attention in paleopathology focused on
these diseases in the historical record. Such classifications tend to
be on the basis of most probable cause and when collections of
skeletal material demonstrating pathology classifications has been re
examined there have been many examples the initial classifications
being challenged. (Picture from Infections in Palaeopathology :
The Basis of classification according to most probably cause. By
Juliet Rogers and Tony Waldron 1989).
Donoghue (2011) describes the gradual
inclusion of bone specimens for TB analysis by DNA techniques that
did not show bone pathology, the initial studies took this as
indications of haematogenous spread of tuberculosis bacilli. There
have been cases where not MTBC aDNA was found in bones demostrating
pathology, most likely attributable to poor DNA preservation but at
least one study has found brucellosis DNA sequences, IS6501 in
vertebra negative for IS6110 and M bovis sequences oxyR pseudogene
and mpt40 that showed pathology that could easily be classified as
typical of M tuberculosis (Mutolo 2011).
Up until the 1960's it was still being
argued that there was no Pre Columbian tuberculosis on the basis of
skeletal material.
The cut off for pre-Columbian contact is
1492 AD, the exact date of the mummy is not given but is around this
time. It is significant because an early theory for the absence of
skeletal remains showing TB in the Americas was that TB was
introduced by European contact, and was still being argued about in
the 1960s by Dan Morse. An interesting part of his argument was that
North Americans lived in small population densities that would not
have supported the disease, making a comparison to then contemporary
indian populations, he calculated that 2.24 percent of skeletal
material should present evidence of bone tuberculosis and 0.67
percent with vertebral involvement (Roberts & Buikstra 2003 :
188).
The study conducted by Corthals et al
(2012), titled “Detecting the immune system response of a 500 year
old inca mummy” uses PCR amplification to detect the presence of
Mycobacterium tuberculosis and “shotgun proteomics” to detect the
protein expression in buccal swabs and cloth samples from 500 year
old Andean mummies, two young children and an adolescent girl.
The mummies were buried 25 meters from
the 6739 metre summit of Llullaillaco, a high elevation volcano in
the province of Salta, Argentina. They were buried 50 cm underground
and packed with volcanic ash. This ash inhibited the growth of
decomposing bacteria & fungi, functioned as a barrier to moisture
and airtight with snow and the freezing temperature, the corpses
were preserved by the combination of mild humidity, anaerobic
environment and natural disinfectants (see figure B).
 |
| Figure B |
The paper describes the three children
as being sacrificed to Pachamama, the earth goddess, in the ritual of
Capacocha. Subcutaneous fat in the bodies was converted to soap by a
process called adipocere.
The presence of pathogenic
Mycobacterium was indicated as being probable by PCR using primers
for the genus Mycobacterium and primers belonging to species in the
Mycobacterium tuberculosis complex. For a lot of DNA techniques the
significance of the presence of Mycobacterium DNA is questionable,
part of the natural soil flora, a contaminant or an indication of the
pathogen. Early work trying to confirm the presence or absence of a
pathogen used a fragment of the M. tuberculosis complex MTBC
specific repeat element, but this is for the whole MTBC complex, not
just Mycobacterium tuberculosis. Because of the good preservation of
the material the study has been able to use a wide range of primers,
including species specific primers, as indicated in this figure
(figure C).
 |
| Figure C |
It has been argued that the
mycobacterium cell wall properties can potentially enhance the
preservation of nucleic acids (Donoghue & Spigelman 2006) but
Wilbur et al 2009 argues that the same cell wall properties will
enhance its degradation, so this is an issue to be resolved. More
recent papers describe the use of a MALDITOF, Matrix Assisted Laser
Desorption Ionization Time of Flight, mass spectrometry technique
that detects the presence of the tuberculosis using the lipid
components of the cell wall, mycolic acids. This is not without its
issues, but it is potentially able to be used to detect Mycobacterium
tuberculosis pathogen in less well preserved skeletal material.
The Corthals et al (2012) paper wanted
to establish whether or not the mummy of the adolescent female had an
active infection at the time of her sacrifice. It would be
interesting to speculate whether this influenced the process of her
being used in the sacrifice. The proteonomics technique used by the
study was able to match up the mass spectrum of proteins from the
lip swab sample of the 15 year old adolescent female to a library of
immune system proteins.
They found normal serum proteins and
some proteins not normally present in blood or saliva but are
consistent with immune response to infectious disease. These
included,
- Cathepsin G, a specialized neutrophilic polymorphonuclear leukocyte serine protease found in the azurophil granules, with its function liked to pathogenesis of diseases associated with inflammation and neutrophil infiltration of the airways.
- Alpha antitrypsin, a marker of chronic lung infection, a strong indicator of mycobacterial infection.
- Neutrophil defensin 1 and 3 are part of the defensin family of cysteine rich cationic proteins found in leukocytes and are specifically associated with macrophages involved in lung tissue inflammation response.
- Proteins including apolipoproteins A1 & A2 and transthyretin, which are expressed in chronic and acute lung inflammation, and can be used as monitoring biomarkers for pulmonary related diseases.
After the proteomics technique the
paper presents a PCR and a maximum likelihood phylogeny to indicate a
higher probability that presence of Mycobacterium belonged to the
pathogenic MTBC complex.
The ancient lineage of Mycobacterium
tuberculosis is suggested from a study on a pre-Pottery Neolithic
site in Atlit Yam in the Eastern Mediterranean dating from around
9250 to 8150 Before present era, bones belonging to a woman and
infant demonstrated morphological changes, the detection of ancient
DNA and MTBC specific cell wall lipid biomarkers (Donoghue 2011).
Helen Donoghue (Donoghue 82 : 2008)
describes the use of cell wall lipid biomarkers for the purpose of
identifying members of the MTBC, with the advantage that the lipid
biomarkers are more stable, potentially lasting longer than DNA and
the methods used, HPLC and Mass spectroscopy are sensitive enough for
direct detection.
There are issues with the use of lipid
biomarkers for identification of members of the MTBC, although the
recent mycoloyl arabinoglactan- peptidoglycan complex is known,
ancient MTBC mycolic acids as synthesized are potentially different
due to adaptive evolution (Mark et al 112 :2011) and will undergo
chemical reactions post mortem thus over time will change the mass
spectrum peaks and fragment identification. The images on the screen
are from Mark Lazlo's et al (2011) paper “Analysis of ancient
mycolic acids by using MALDI TOF MS: response to “Essentials in the
use of mycolic acid biomarkers for tuberculosis detection” by
Minnikin et al 2010. Lazlo is referenced by Helen Dononhue (2011) in
the detection of lipid biomarkers, the team is responding to a
critique that the method is not sensitive enough for its application
for archaeological material.
When it was being argued that there was
no MTBC on the American continent, part of this argument was there
was a founder effect bottleneck and a low population density did
support endemic Mycobacterium tuberculosis. The low population
density argument is shown to be flawed with evidence of high density
sedentary Pre-Columbian populations in North America, without even
discussing Meso and South America but also a different picture of the
incidence of Mycobacterium tuberculosis in ancient American
populations is emerging. Because of M tuberculosis biomarkers are
being found in archaeological material without demonstrating
pathological change the incidence of M tuberculosis in historical/
paleolithic populations is seen to be greater than previously
estimated (Donoghue 821 : 2011). This is accompanied by more recent
insights in the origin and evolution of M tuberculosis.
A paper by Holloway et al (2011) titled
Evolution of human tuberculosis: A systematic review and
meta-analysis of paleopathological evidence attempts to compare
all known cases of TB showing bone involvement from the range of 7250
BCE to 1899 and does a Chi squared test against the null hypothesis
that the frequency and location of bone lesions does not change over
time. The papers data was reported cases in literature, which yielded
530 TB cases from 221 gravesites reported in 151 references (Holloway
et al 407 : 2011). One of the interesting aspects of the paper was
its classification of sites in terms of pre urbanised, urbanised and
early modern time periods. In the Mediterranean early urbanization
is associated with Phoenician and Greek expansion, the beginning
date used was from 600 BCE (before the common era), while in Northern
Europe early urbanization is from 800 CE (common era). The paper
classifies early urbanization in the Americas as from 1500 CE, the
point of European contact. The paper produced a bar chart, comparing
time periods that shows a decrease in the frequency of skeletal
lesions over time, and a decrease in the frequency of involvement of
the spine with an increased involvement in other skeletal locations
(Holloway et al 411 : 2011). The significance of this can be
discussed with respect to an “osteological paradox” (Holloway et
al 414 : 2011), where to develop bone lesions the individual has to
be generally immunologically resistant enough and healthy enough to
survive a chronic condition. Thus this decrease in the frequency of
bone lesions could mean that the frequency of individuals with
sufficient immunological resistance has decreased. They argue that
because the average age of death was the same in all three time
periods that this is unlikely and describe a general evolutionary
process of decreased virulence of bacteria over time, although the
cause of death may not have been due to TB.
 |
| Figure D |
This decreased virulence over time is
argued to be evidence of a form of sympatric speciation, a process of
coevolution that is often demonstrated by reciprocal transplant
experiments, where the performance of locally adapted pathogen
variants (sympatric) are compared to non locally adapted pathogen
variants (allopatric) ( Fenner et al 2 : 2013). An indirect way to
observe this is to look at the performance of pathogen variants when
the immune system of individuals of the sympatric host population is
disrupted. A paper by Fenner, Egger, Bodmer, Furrer, Ballif et al
2013 called HIV infection disrupts sympatric host pathogen
relationships in human tuberculosis has done this presenting a
statistical argument that HIV infection disrupts sympatric host
pathogen relationships in human tuberculosis. Currently Mycobacterium
exists in six main phylogenetic lineages associated with geographical
regions with sympatric human populations. Transmission tends to occur
in higher frequency in sympatric host pathogen combinations and these
associations remain in international cosmopolitan situations. The
Fenner et al study focused on 233 European born patients from a
population of 518 patients (21.6% had HIV) and defined a sympatric
host-pathogen relationship as infection with lineage 4 and allopatric
as infection with any other lineage. From this group of 233 European
born patients, 36 had HIV and of these individuals 9 had an infection
with a non lineage 4 Mycobacterium tuberculosis strain (Fenner et al
4 : 2013).
 |
| Figure E |
Using a multivariate analysis there was a statistically
significant associated between HIV infection and infection by
allopatric M tuberculosis (unadjusted OR 7.0, 95% Confidence interval
2.5 -19.1, p value less than 0.0001). It also found that the strength
of the association between HIV infection and allopatric lineage
increased as CD4 T cell count went down (Fenner et al 6 : 2013). The
study has attempted to adjust for age, sex, frequency of travelling,
contact with foreign populations and other forms of immunosuppresion
and found that the only strong adjusted variable was repeated
travelling to low income countries with a decreased OR to 4.5 (95% CI
1.5 -13.6, p = 0.008). This data supports the idea of co-evolution
of the Mycobacterium tuberculosis and human host populations.
The time scale for the co evolutionary
relationship of the Mycobacterium tuberculosis Complex with humans is
suggested by genome sequencing of M tuberculosis to be at least 2.6
to 2.8 million years ago, earlier than the range of Homo habilis (2.3
to 1.4 million years ago), suggested by synonymous sequence diversity
(Donoghue 825 : 2011), there is a possible material evidence of case
of TB in Homo erectus dating to 490 -510 000 years ago, from a
partial skeleton found in western Turkey (Donoghue 826 : 2011) .
The common image of Mycobacterium
tuberculosis, with very good reason, is as a scourge of high density
population centers characterised by the conditions of the early
industrial revolution (20th century) and urban populations
characterised by poverty. It is the medical reasoning for
antispitting laws that were enforced by New York City around 1910,
recently Waltham Forest Council in Britain (Paul Cocozza, from The
Guardian, September 2013) fined spitters and there is currently
an anti spitting Bill is being introduced in the Phillipines. But
given its long association with humans, predating the Neolithic, the
predominant conditions providing the evolutionary selection pressure
on it would be different from those now.
These pre Neolithic conditions would be
low density populations, that are composed of small, groups organised
around kinship structures that are predominantly mobile (Donoghue 826
: 2011). Because of this the organism would be under selection
pressure to survive through the lifetime of the individual host, with
transmission occurring from adults with reactivation of the disease
to other individuals, including infants with immature immune systems.
Reactivation would occur in situations of nutritional deprivement,
age related failing immune systems, and physical and mental stress
(Donoghue 826 : 2011). As such the organism would be selected towards
a quasi-commensal host pathogen relationship, with increased latency
in the majority of cases, over time there would be co evolutionary
processes leading to sympatric host pathogen relationships.
Conclusions
One of the insights that this model
provides is that given the increased population density, with a
greater opportunity for transmission, the selection pressures
favoring latent infection would be reduced and so selection pressures
should favor an increased bacterial virulence (Donoghue 827 : 2011).
There is evidence of evolutionary modern lineages that induce lower
levels of early inflammatory response and faster progression to
active disease (Donoghue 827 : 2011).
The use of antibiotics is a selection
factor, and most papers describe the mutation rates of Mycobacterium
tuberculosis in resistance to line 1 antibiotics, isoniazid,
rifampicin and ethambutol as within the range of 1 X 10^-7
(ethambutol) to 2.3 X 10^-10 (rifampicin) and seem to describe
Mycobacterium tuberculosis as predominantly transferring antibiotic
resistance vertically, through generations (Gumbo 722 : 2013). In
lineage 2, which includes the Beijin strains, there is a prevalence
of MDR tuberculosis. It has been found that lineage 2 appears to have
a tenfold increased acquisition of rifampicin resistance compared to
lineage 4. This suggests another implication, that there are
different rates of multidrug resistance (antibiotic resistance) in
the different lineages.
 |
| Figure F |
Comments.
Rabbit test models to determine when
treponemal dissimination found that in later stages of the disease
Treponomal DNA could not be isolated from the bone. Syphilis produces
marks of severe inflammation on long bones and cranium, deep erosions
and nodes that can be described as a moth eaten appearance and a
thickening of the long bones. Sectioned bone reveals a spongy
appearance with obliteration of the medullary cavities.
Holloway et al (2011) uses Pompeii as a
way of checking whether the frequency of lesions in the age and sex
distributions found in the cemeteries corresponded with the frequency
of lesions in living population age and sex distributions. It was
found that it did not differ in adult populations but did differ
significantly in sub adult (child) populations (Holloway et al 414 :
2011), this is why the Holloway et al (2011) paper only discusses
adult populations.
Mechanisms of action of antibiotics, for figure F.
Ethambutol inhibits arabinosyl
transferase involved in cell wall biosynthesis, the enzyme is coded
for by three homologous genes embCAB and resistance is associated
with mutation of these loci
Isoniazid is a peroxide producing drug
that relies on catalase activity to interfere with mycolic acid
biosynthesis.
Rifampic prevents transcription of DNA
dependant RNA polymerase, binds to beta subunit, resistance is
conferred by mutations of the rhoB gene that encodes
Pyrazinamide interferes with fatty acid
synthesis, lowering intracellular pH which inactivates fatty acid
synthase
Bibliography
Donoghue, Helen D. (2008). Chapter 6 :
Palaeomicrobiology of Tuberculosis. In D Raoult & M Drancourt
(eds). Paleomicrobiology : Past Human Infections. Published by
Springer-Verlag Berline Heidelberg. Pages 75 -97
Donoghue, H.D. (2011). Insights gained
from palaeomicrobiology into ancient and modern tuberculosis. In the
Journal of Clinical Microbiology Infectious Diseases. Volume
17. Pages 821 – 829.
Fenner, L; Egger, M; Bodmer, T; Furrer,
H; Ballif, M et al. (2013). HIV Infection Disrupts the Sympatric
Host-Pathogen Relationship in Human Tuberculosis. In PloS Genet
Volume 9, Index 3. e1003318. doi:10.1371/journal.pgen.1003318
Gagneux, Sebastien. (2012).
Host-pathogen coevolution in human tuberculosis. In Philosphoical
transactions of The Royal Society. Volume 367. Pages 850 -859.
Gumbo, Tawanda. (2013). Biological
variability and the emergence of multidrug-resistant tuberculosis. In
Nature Genetics. Volume 45. Pages 720 -721.
Holloway, K.L; Henneberg, R. J; Lopes,
M de Barros & Henneberg, M. (2011). Evolution of human
tuberculosis: A systematic review and meta-analysis of
paleopathological evidence. In Homo- Journal of Comparative Human
Biology. Volume 62. Pages 402 -458.
Iseman, Michael. (1994). Evolution of
drug resistant tuberculosis: A tale of two species. In Proceedigns of
the National Academy of Sciences of the United States of America .
Volume 91. Pages 2428 -2429.
Mutolo, Michael ; J. Jenny, Lindsey;
Buszek, Amanda; Fenton, Todd W & Foran, David. (2012).
Osteological and Molecular Identification of Brucellosis in Ancient
Butrint, Albania. In the American Journal of Physical
Anthropology. Volme 147. Pages 254 -263.
No comments:
Post a Comment